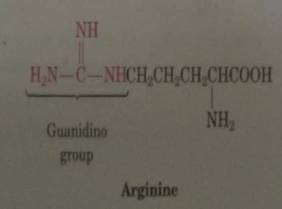
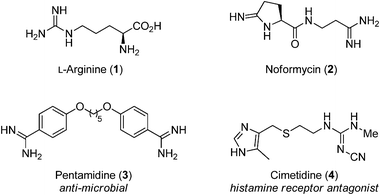
The guanidine group of the amino acid arginine is ubiquitous in Nature In its protonated state, several resonance forms that delocalize the cationic charge of the guanidinium cation over the entire functional group contribute to the high basicity of guanidine (pK aH = 136 in water) 1 The Hbonding ability ofGuanidine is one of the most versatile functional groups in chemistry;Abstract Here we applied a novel method1a to predict pKa values of the guanidine functional group, which is a notoriously difficult This method, which was
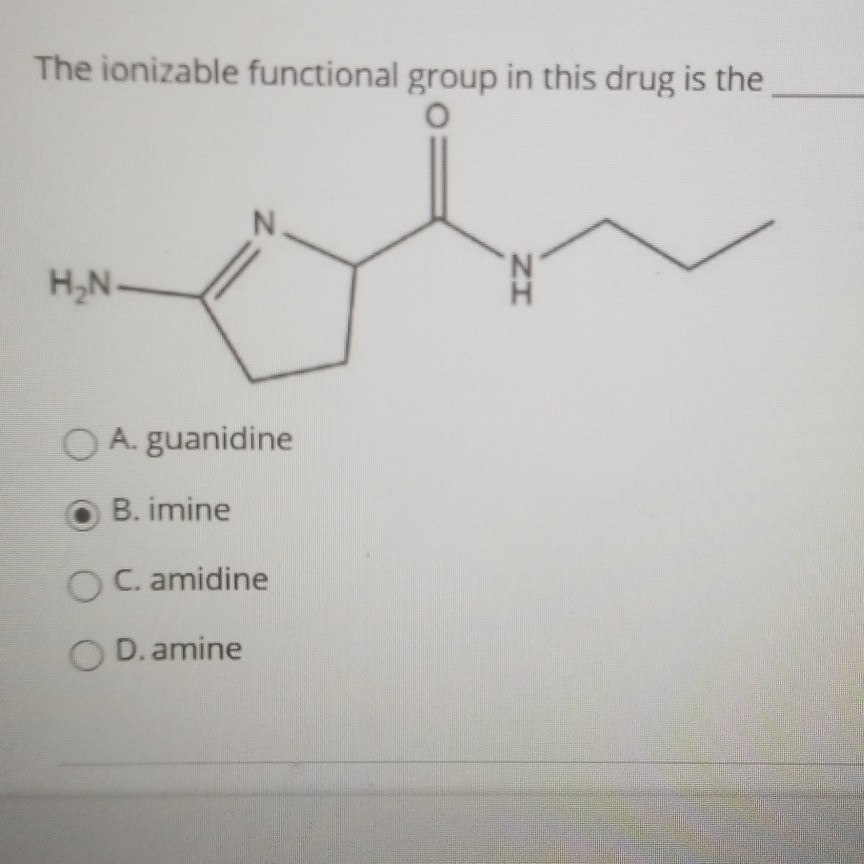
Solved The Ionizable Functional Group In This Drug Is The O Chegg Com
Guanidine functional group
Guanidine functional group-Guanidino group ( CHEBI ) is a organoheteryl group ( CHEBI ) guanidino group ( CHEBI ) is substituent group from carbamimidoylazanium ( CHEBI ) guanidino group ( CHEBI ) is substituent group from guanidine ( CHEBI4 ) Incoming 1dodecylguanidine ( CHEBI740 ) has part guanidino group ( CHEBI)Introduction Compounds incorporating guanidine moiety have found many practical applications in diverse areas of chemistry, such as nucleophilic organocatalysis, anion recognition and coordination chemistry Moreover, guanidine functional group is found in natural products, pharmaceuticals and cosmetic ingredients produced by synthetic methods




Pdf Amidines Isothioureas And Guanidines As Nucleophilic Catalysts Semantic Scholar
Guanidine is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions(2) Guanidine does not contain chromophores that absorb at wavelengths >290 nm and therefore is not expected to be susceptible to direct photolysis by sunlight(2)Turner Construction Company Aug 13 Jun 151 year 11 months Dallas/Fort Worth Area Project Hall Arts Center Downtown Dallas ($72 million)Lipoplexes of DNA and siRNA 68 The polar (cationic) headgroup can be an amine (primary, secondary, tertiary, and even quaternary eg, imidazolium 9) or guanidine functional group Guanidines, the most basic functional group in biological chemistry, are positively charged at physiological pH 74 as they have pKa = 125 10 Guanidines
Guanidine and Functional group See more » Guanidine nitrate Guanidine nitrate is the chemical compound with the formula NO3 New!!Arginine R (Arg) Arginine, an essential amino acid, has a positively charged guanidino group Arginine is well designed to bind the phosphate anion, and is often found in the active centers of proteins that bind phosphorylated substrates As a cation, arginine, as well as lysine, plays a role in maintaining the overall charge balance of a proteinConsidered to have acidic character At pH=1, Pepcid's guanidine group will be ionized in the stomach (pKa=105;
The use of the guanidine functional group to connect monosaccharide units in glycooligomers is particularly attractive with regard to molecular recognition processes Like thioureas and ureas, guanidines can also form bidentate hydrogen bonds In addition, because of their positively charged character, guanidines can exert strong electrostaticIn one embodiment, the bicyclic guanidine compound may be chosen from a compound of Formulas (2)(9) or a combination of two or more thereof One or more different guanidinecontaining compounds with a guanidine functional group as part of a fused ring system may be used as the catalyst material in the ring opening polymerization process The multidendate binding capabilities of the guanidine functional group A high concentration of guanidines can also assist with translocation across membranes 11, 15–17 thus underlying the fact that polyarginine functions as a cellpenetrating peptide 18–



Molecules Free Full Text A Synthetic Method To Access Symmetric And Non Symmetric 2 N N Disubstituted Guanidinebenzothiazoles Html




Designed Guanidinium Rich Amphipathic Oligocarbonate Molecular Transporters Complex Deliver And Release Sirna In Cells Pnas
Here, we report on a proteasefocused DECL and DECL screening strategies designed to engage the protease catalytic triad Since many of the known protease inhibitors contain guanidine, sulfonamide, urea, and carbamate moieties, we incorporated these functional groups into the design of this library (26, 27)By the urea cycle in bacteria and differs from guanidine by only a single functional group, where one amine (CNH 2) in guanidinium is a carbonyl group (C=O) in urea (Nelson et al, 16) The consensus motif for the guanidine riboswitch generated by bioinformatics provides less structural information thanStart studying Functional Group Learn vocabulary, terms, and more with flashcards, games, and other study tools Home Subjects Subjects Languages View all English French German Latin Spanish Guanidine Basic functional group (polar) pKa= 12 for conjugate acid) Other sets by this creator Therapeutic Category 4 31 terms




Antimicrobial Drugs Bearing Guanidine Moieties A Review Sciencedirect




Guanidine Png Images Pngwing
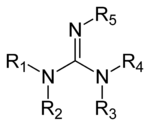
ABSTRACT The guanidine functional group is an important structural motif in synthesis with a wide range of interesting properties Guanidines are frequently found in bioactive compounds;Guanidine derivatives Guanidines are a group of organic compounds sharing a common functional group with the general structure (R 1 R 2 N)(R 3 R 4 N)C=NR 5 The central bond within this group is that of an imine;Cimetidine ChEBI ID CHEBI3699 Definition A member of the class of guanidines that consists of guanidine carrying a methyl substituent at position 1, a cyano group at position 2 and a 2 { (5methyl1 H imidazol4yl)methylsulfanyl}ethyl group at position 3 It is a H 2 receptor antagonist that inhibits the production of acid in stomach




Amines The Organic Bases Categorizing Amines N Amines




Guanidine And Guanidinium Cation In The Excited State Theoretical Investigation The Journal Of Chemical Physics Vol 141 No 7
Compounds containing this system have found application in a diversity of biological activities, and in this chapter, theThe germicidal function is attributed to the ability of the guanidine functional groups of the Teflex polymer bonding with the cellular membranes of pathogenic microbes Upon bonding, the guanidine functional group infiltrates the cells cytoplasm and The guanidineI riboswitch is a conserved RNA element with approximately 2,000 known examples across four phyla of bacteria It exists upstream of nitrogen metabolism and multidrug resistance transporter genes and alters expression through the specific recognition of a free guanidinium cation Every functional group of the ligand is
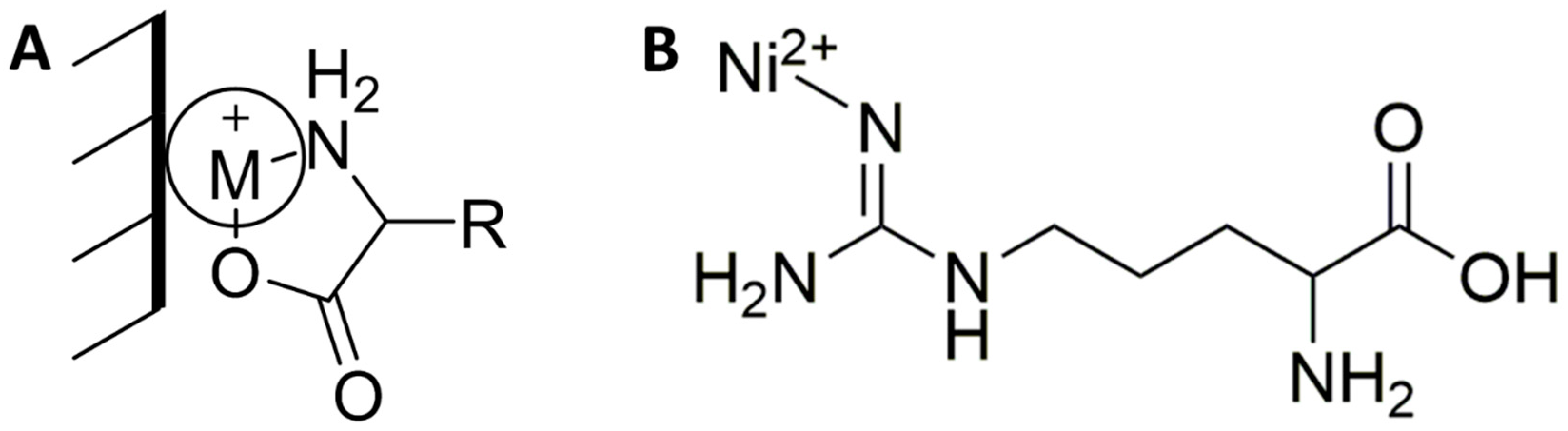



Sensors Free Full Text Evaluation Of Metal Oxide Surface Catalysts For The Electrochemical Activation Of Amino Acids Html



Arginine Which Contains A Guanidine Functional Group In I Chegg Com
PKa Data Compiled by R Williams ACIDS Compound pK Ref H3PO2 , 223* 28 H2PO4– 721* 77 AgOH 396 4 HPO4_ 1232* 77 Al(OH)3 112 28 As(OH) H3PO3 28 3 922 28 H3AsO4 222, 70, 130 28 H2PO3– 658* 77Density functional theory 1 Introduction A major topic in bioinorganic chemistry is the analysis, reproduction, and, ultimately, the improvement of the active sites of natural catalytic systemsIncorporation of a guanidine functional group into the PNA backbone facilitates cellular uptake of PNA into mammalian cells with efficiency comparable to that of the TAT transduction domain The modified PNA recognizes and binds to the complementary DNA strand in accordance with Watson−Crick recognition rules However, unlike polypyrimidine PNA which binds to DNA in 21




General Structure And Synthesis Of The Guanidinium Rich Amphipathic Download Scientific Diagram




Concise Synthesis Of Guanidine Containing Heterocycles Using The Biginelli Reaction Abstract Europe Pmc
Interface with customers in solving quality issues and proactive continuous improvementsFrequently interact with customers and TI functional group managers and engineers inEnhanced drug toxicity by conjugation of platinum drugs to polymers with guanidine containing zwitterionic functional groups that mimic cellpenetrating peptides K J Abd Karim, R H Utama, H Lu and M H Stenzel, Polym Chem, 14, 5, 6600 DOI /C4PYBDine or guanidine functional groups built onto aromatic or heterocyclic scaffolds (Figure 4) 1216 A major limitation to the use of these inhibitors is their poor bioavailability, which is at least partly owing to the presence of the positively charged amidine or guanidine group This has limited clinical studies of these uPA inhibitors
/63589E4AF83B1572802585F9006F4B6A/$file/FG09296_structure.png)



Guanidine Hcl 50 01 1 Biosynth Carbosynth



Recent Advances In Guanidine Based Organocatalysts In Stereoselective Organic Transformation Reactions Intechopen
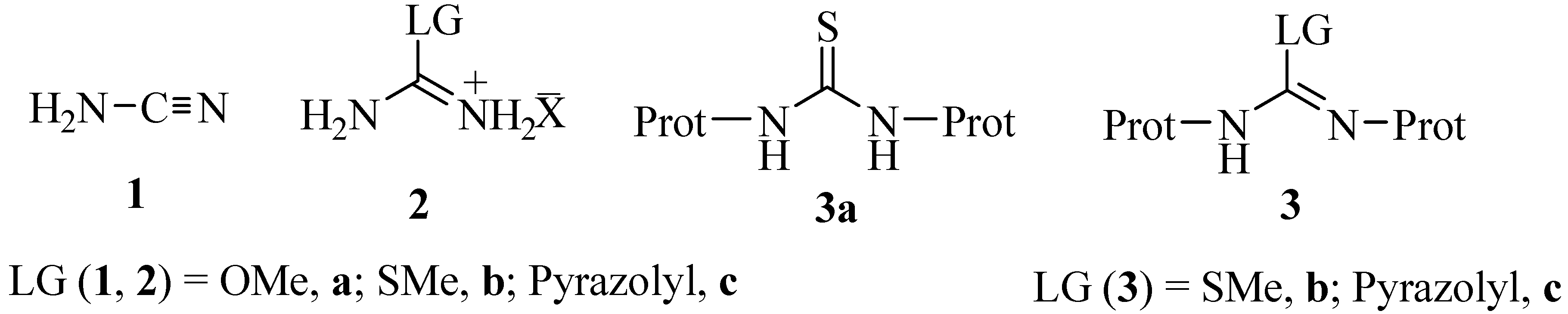
This paper relates how 21 (N,N'bisBocN''triflylguanadine), with its NTf group and two Boc protecting groups, reacts with a deprotected amine to install a guanidine functional group on Specifically, the article states that in order to add the guanidine group, triethylamine (TEA) is necessary, indicating that the mechanism involvesHere we applied a novel method 1a to predict pK a values of the guanidine functional group, which is a notoriously difficult This method, which was developed in our lab, uses only one ab initio bond length obtained at a low level of theory The method is shown to work for drug molecules, delivers prediction errors of less than 05 log unitsThe other recognizable motif within this group is an aminal




Structure Activity Relationships Of Guanylated Antimicrobial Polymethacrylates




Derivatives Of The Triaminoguanidinium Ion 6 Aminal Forming Reactions With Aldehydes And Ketones
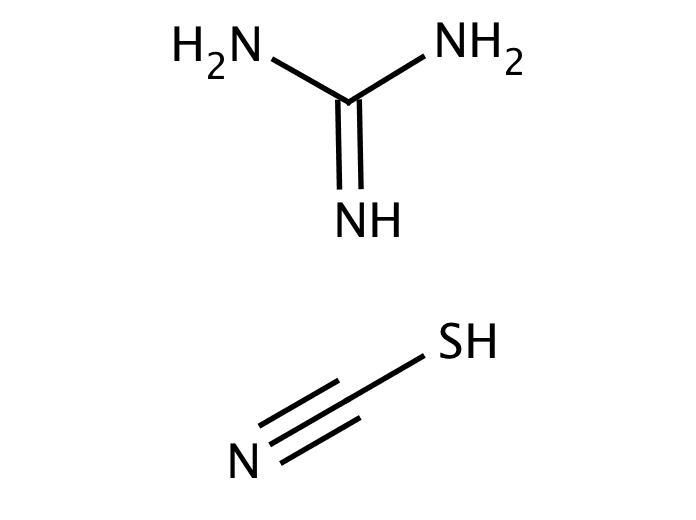
Guanidine, also called carbamidine, is a strongly alkaline and watersoluble compound, NHC (NH 2) 2 It is formed in urine as a normal product of protein metabolism in the body by the oxidation of guanine Guanidine has imine group and aminoacetal functional group in the small structure Aminoacetal (aminal) is the functional group which has twoThis process for the synthesis of 3mercaptopropionic acid by an addition reaction of H 2 S with acrylic acid is carried out in the presence of a solid support having basic guanidine functional groups, provided that the latter do not contain hydrogen bonded directly to a nitrogen atomAcidity of amidine and guanidine conjugation, Nhydroxylation 2 metabolic reactions of amidine and guanidine carboxylic acid name functional group acidic name functional groups (left to right) basic acidity of indole neutral acidity of benzofuran neutral acidity of benzothiophene benzimidazole name functional group basic



Guanidine As Inexpensive Dual Function Ligand And Reducing Agent For Atrp Of Methacrylates Polymer Chemistry Rsc Publishing




Solved The Ionizable Functional Group In This Drug Is The O Chegg Com
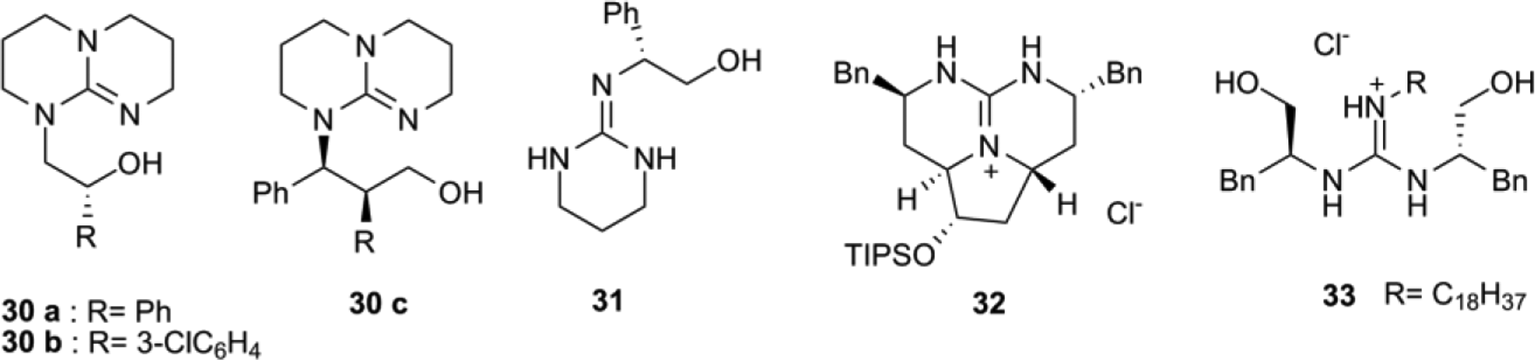
The guanidine functional group is found in many biologically active products, making it a worthwhile chemical target To this end, strained, tertiary, allylic, amine 2benzyl2azabicyclo221hept5ene reacts with insitu generated carbodiimides in the 1,3diazaClaisen rearrangement to afford structurally interesting bicyclic guanidines With the aim to apply symtetrazines (1, 2, 4, 5 tetrazines) in catalystfree functionalizations of norbornenes (bioorthogonalizations) and preparation of functional organic materials (fullerenes), a systematic computationational study was carried out In particular, the cycloaddition properties of novel 2pyridylsym tetrazines bearing a guanidine functional groupPossesses two guanidine groups as streptomycin, dihydrostreptomycin, rtiethylenetetramine, bs3ai minoproyalmine, guanidine hydrochloride, and spermine tetrahydrochloride The presence of these 2 functional guanidine groups within a nonpolymeric hydrophilic molecular system was suspected to be the chemical structure of streptomycin



Amidines Isothioureas And Guanidines As Nucleophilic Catalysts Chemical Society Reviews Rsc Publishing Doi 10 1039 C2cs152f




Pdf Guanidine Group Definition And Pharmaceutical Applications
The new treatment accounts for multiple conformations both on the level of energetics and parametrization Illustrative results are shown for several types of chem structures contg guanidine, amidine, amine, and phenol functional groups, and which are representative of practically important large and flexible druglike mols Calculations indicate the nitro group of nitroguanidine dramatically attenuates the basicity of the guanidine functional group The large increase in the proton affinity for protonation at the guanidine site for desnitroimidacloprid vs imidacloprid drives the loss of NO 2 upon CID of protonated imidacloprid, which is inhibited for the other ionsThe guanidine functional group, displayed most prominently in the amino acid arginine, one of the fundamental building blocks of life, is an important structural element found in many complex natural products and pharmaceuticals Owing to the continual discovery of new guanidinecontaining natural p



Purchase Guanidine Thiocyanate 593 84 0 Online Katalog Molekula Group



Guanidine Wikipedia
Chloride, Guanidinium Chloride, Guanidium Guanidine Guanidine Hydrochloride Guanidine Monohydrate Guanidine Monohydrobromide Guanidine MonohydrochlorideGuanidinebased functional groups occur in many branches of chemistry, due in part to their ability to exist as neutral (guanidine), cationic (guanidinium), and anionic (guanidinate) entitiesBoth from natural sources or from synthetic origin, these compounds are considered fundamental entities in medicinal chemistry




Antimicrobial Drugs Bearing Guanidine Moieties A Review Sciencedirect




Bestand Guanidine Group 2d Skeletal Png Wikipedia
Thus, the combination of a guanidine functional group and tertiary amine in EDG allows for a higher tolerance towards FFA content Thus, the facile synthesis procedure for the catalysts developedThe general structure of a guanidine Guanidines are a group of organic compounds sharing a common functional group with the general structure (R 1 R 2 N)(R 3 R 4 N)C=N−R 5 The central bond within this group is that of an imine, and the group isFunctional group In organic chemistry, functional groups are specific substituents or moieties within molecules that are responsible for the characteristic chemical reactions of those molecules New!!



Recent Advances In Guanidine Based Organocatalysts In Stereoselective Organic Transformation Reactions Intechopen




Recent Advances In Chiral Guanidine Catalyzed Enantioselective Reactions Chou 19 Chemistry 11 An Asian Journal Wiley Online Library
Barrier Estimates of Guanidine Reactive Site with Guanidinium Anchor Site The neutral guanidine functional group is also reactive with NHS reagents (Scheme 1b) DFT calculations analogous to those conducted for the amine/ammonium system described above were performed to estimate key barriers associated with guanidineIan A Cliffe, in Comprehensive Organic Functional Group Transformations, 1995 (iii) NAcyliminocarbonyl derivatives from guanidines Unsubstituted and monosubstituted guanidines are acylated by condensation of the requisite guanidine free base with the appropriate acid ester < 61CJC1017, 75AF1477 > The preparation of diand trisubstituted guanidines takes longer and inThe pH of the environment is less than the pKa of the basic drug, therefore the functional group will be ionized) At pH=35, the guanidine group will




Pdf Guanidine Motif In Biologically Active Peptides Semantic Scholar




Derivatives Of The Triaminoguanidinium Ion 4 O Sulfonylation Of N N N Tris Hydroxybenzylidenamino Guanidinium Ions



The Chemistry And Biology Of Organic Guanidine Derivatives Natural Product Reports Rsc Publishing



Guanidine Group Guanidine Functional Group 約ネバ エマ アニメ画像



Enamine Guanidine Enol Ether Functional Group Organic Chemistry Png 918x594px Enamine Aldehyde Alkene Amine Area Download



Guanidine A Simple Molecule With Great Potential From Catalysts To Biocides And Molecular Glues




Selective Functional Group Transformation Using Guanidine The Conversion Of An Ester Group Into An Amide In Vinylogous Ester Aldehydes Of Imidazole Sciencedirect



Cimetidine A Rational Approach To Drug Design By




Antimicrobial Drugs Bearing Guanidine Moieties A Review Sciencedirect



Mechanistic Considerations Of Guanidine Catalyzed Reactions Chemical Communications Rsc Publishing




Concise Synthesis Of Guanidine Containing Heterocycles Using The Biginelli Reaction Abstract Europe Pmc



Chiral Guanidines And Their Derivatives In Asymmetric Synthesis Chemical Society Reviews Rsc Publishing




Arginine Mimetics With Reduced Basicity The Additional Functional Download Scientific Diagram



2




Usb2 Antipathogenic Guanidinium Copolymer Google Patents
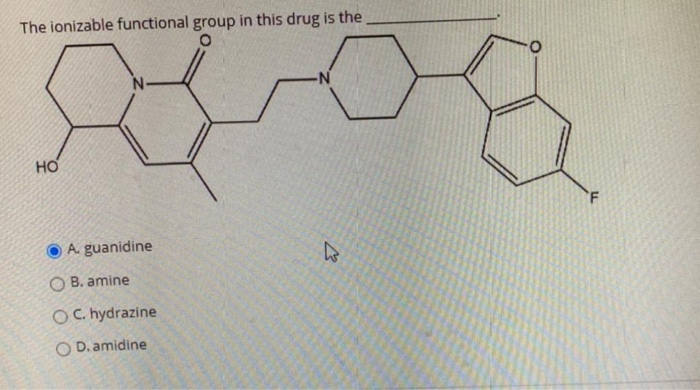



Solved The Ionizable Functional Group In This Drug Is The Chegg Com
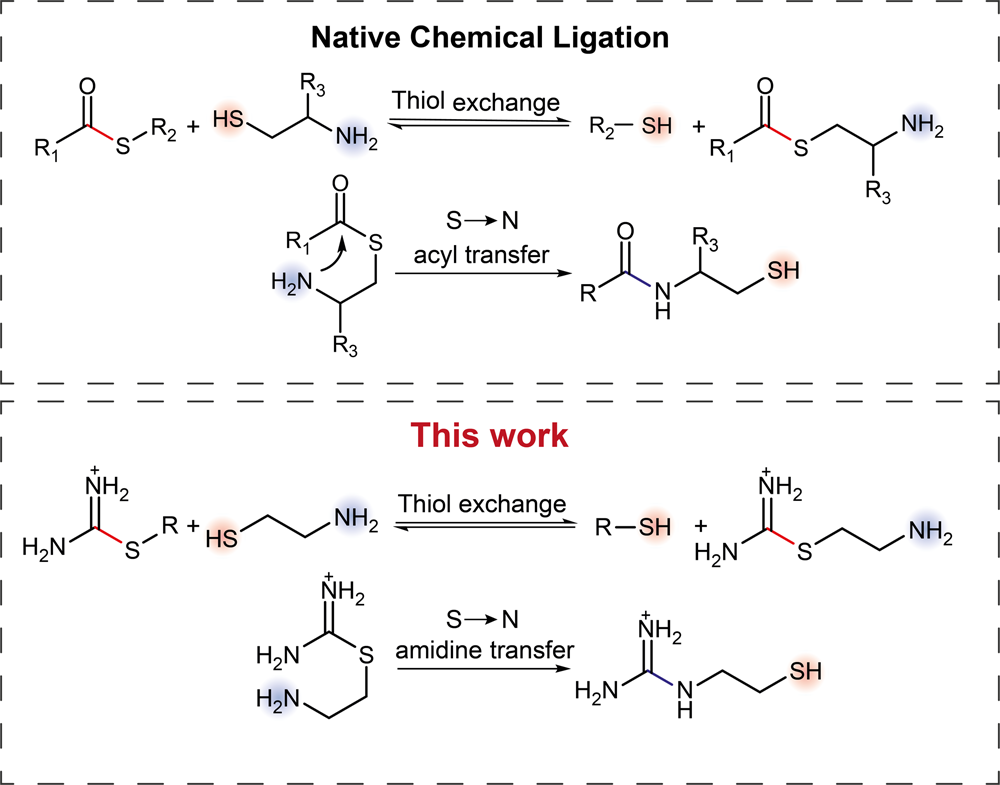



Autocatalytic And Oscillatory Reaction Networks That Form Guanidines And Products Of Their Cyclization Nature Communications




Unusual Oxidative Chemistry Ofn W Hydroxyarginine And N Hydroxyguanidine Catalyzed At An Engineered Cavity In A Heme Peroxidase Sciencedirect
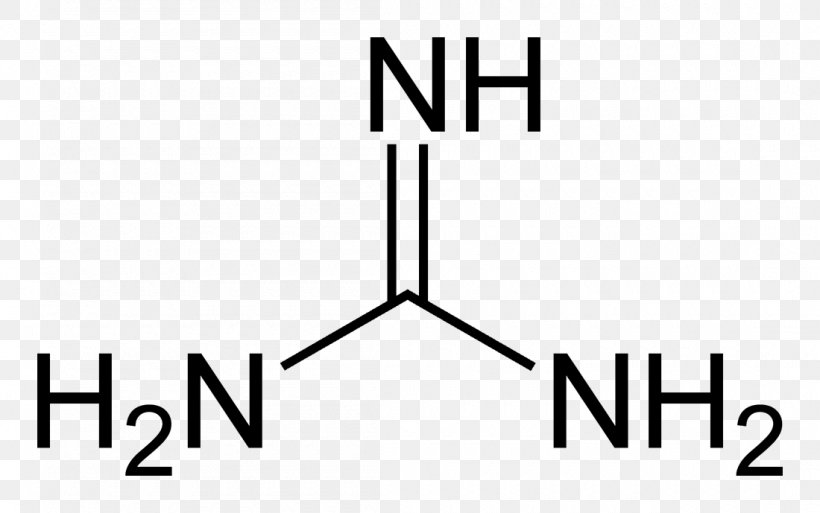



Guanidine Guanidinium Chloride Arginine Hydrochloride Barytwasser Png 1100x6px Guanidine Area Arginine Biguanide Brand Download Free
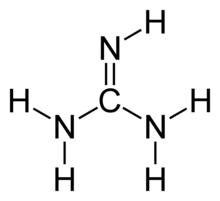



Guanidine Wikiwand
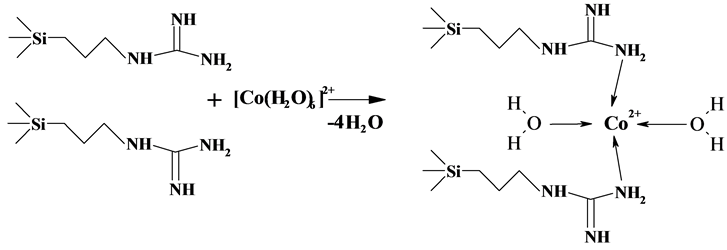



New Approach To Synthesis Of Silica With Chemically Bound Guanidine Hydrochloride For Preconcentration Of Metal Ions
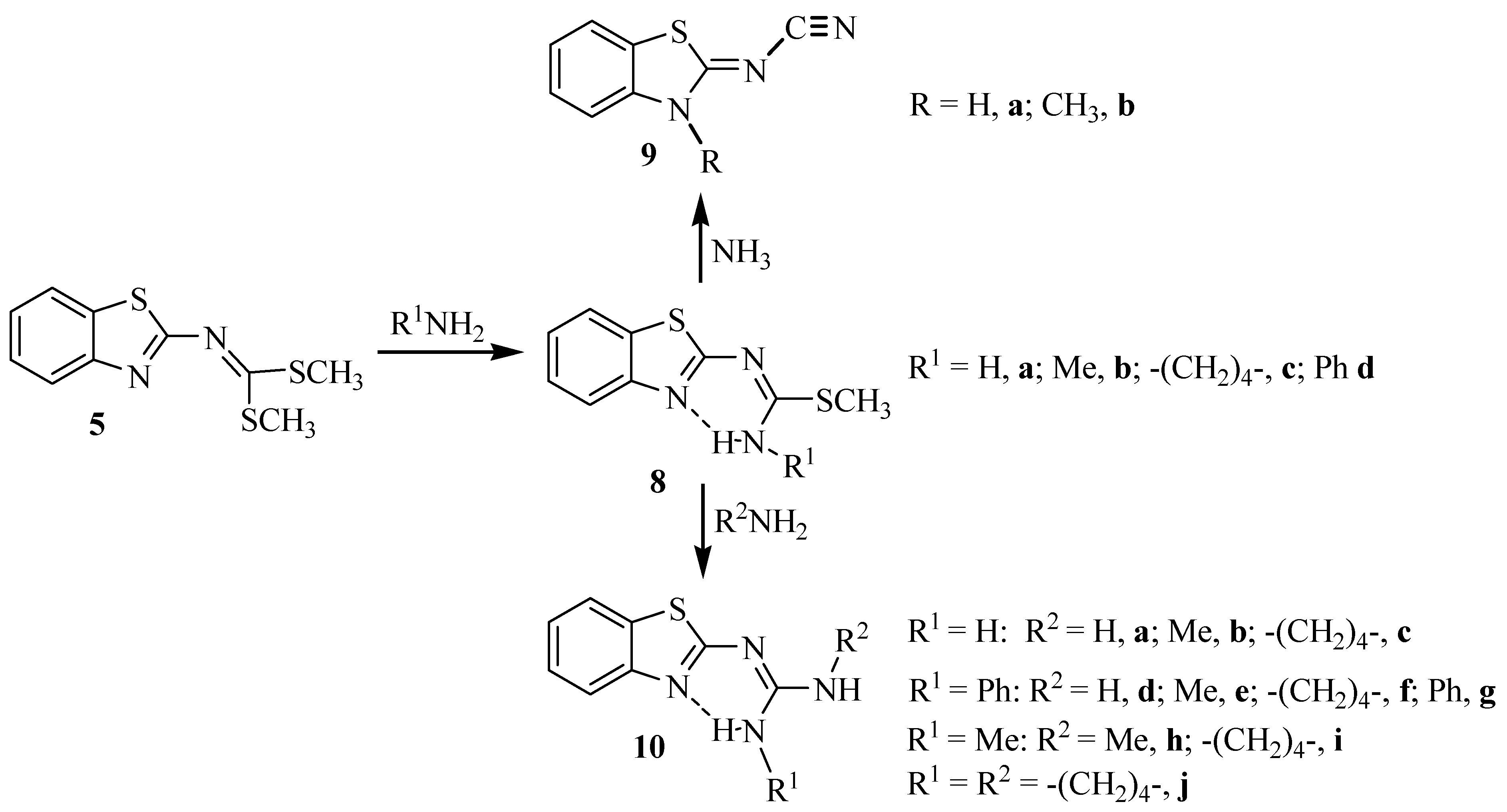



Molecules Free Full Text A Synthetic Method To Access Symmetric And Non Symmetric 2 N N Disubstituted Guanidinebenzothiazoles Html



Guanidine Ligand Page Iuphar Bps Guide To Pharmacology



Guanidine A Simple Molecule With Great Potential From Catalysts To Biocides And Molecular Glues



Impact Of Surface Active Guanidinium Tetramethylguanidinium And Cholinium Based Ionic Liquids On Vibrio Fischeri Cells And Dipalmitoylphosphatidylcholine Liposomes Scientific Reports
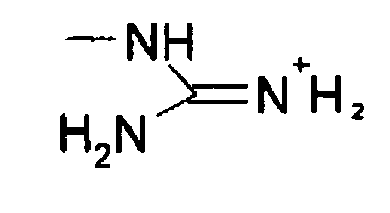



Functional Groups To Know Flashcards Chegg Com




Guanidine Alchetron The Free Social Encyclopedia
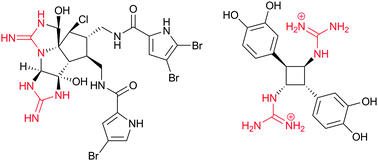



The Chemistry And Biology Of Organic Guanidine Derivatives Natural Product Reports Rsc Publishing




Classical Guanidine Synthesis Guanidine Core Structure Obtained By Download Scientific Diagram




Guanidine Formula Uses Facts Britannica



1




Pdf Guanidine Motif In Biologically Active Peptides Semantic Scholar




Guanidinium Group A Versatile Moiety Inducing Transport And Multicompartmentalization In Complementary Membranes Sciencedirect




Recent Advances In Guanidine Based Organocatalysts In Stereoselective Organic Transformation Reactions Intechopen




Tryr Inhibitors Containing Guanidine Biguanide Or Bis Amidine Download Scientific Diagram
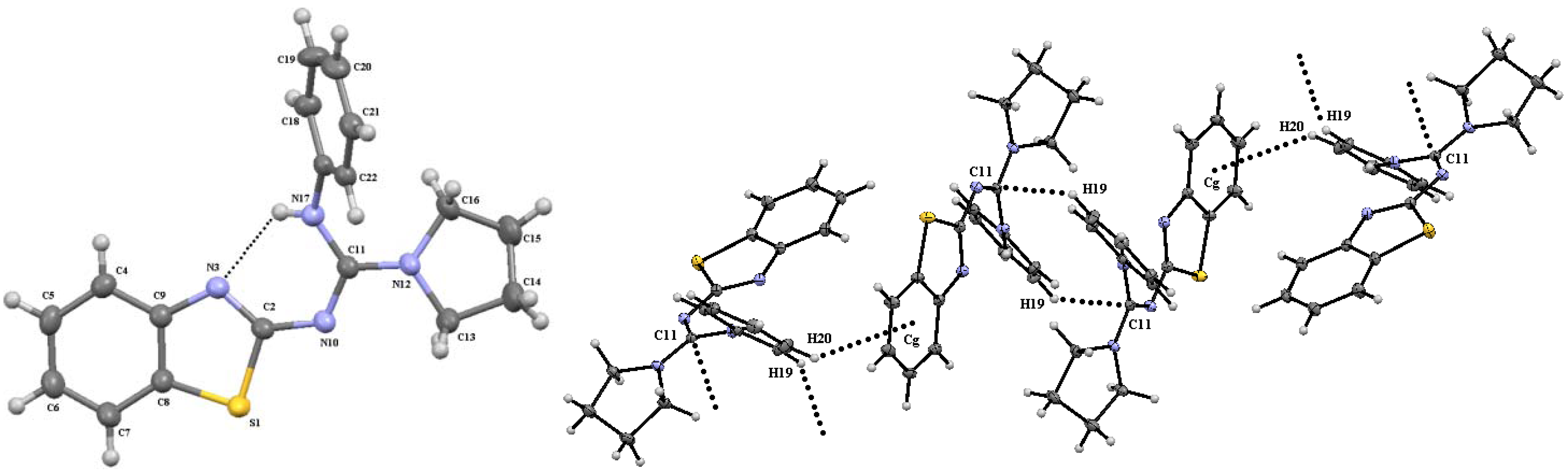



Molecules Free Full Text A Synthetic Method To Access Symmetric And Non Symmetric 2 N N Disubstituted Guanidinebenzothiazoles Html




Bestand Guanidine Group 2d Skeletal Png Wikipedia




Guanidine Phosphate 5423 23 4 Tokyo Chemical Industry Co Ltd Apac




Structure Of Guanidine Tbd 4 Left Its Corresponding Guanidinium Download Scientific Diagram



Guanidine A Simple Molecule With Great Potential From Catalysts To Biocides And Molecular Glues
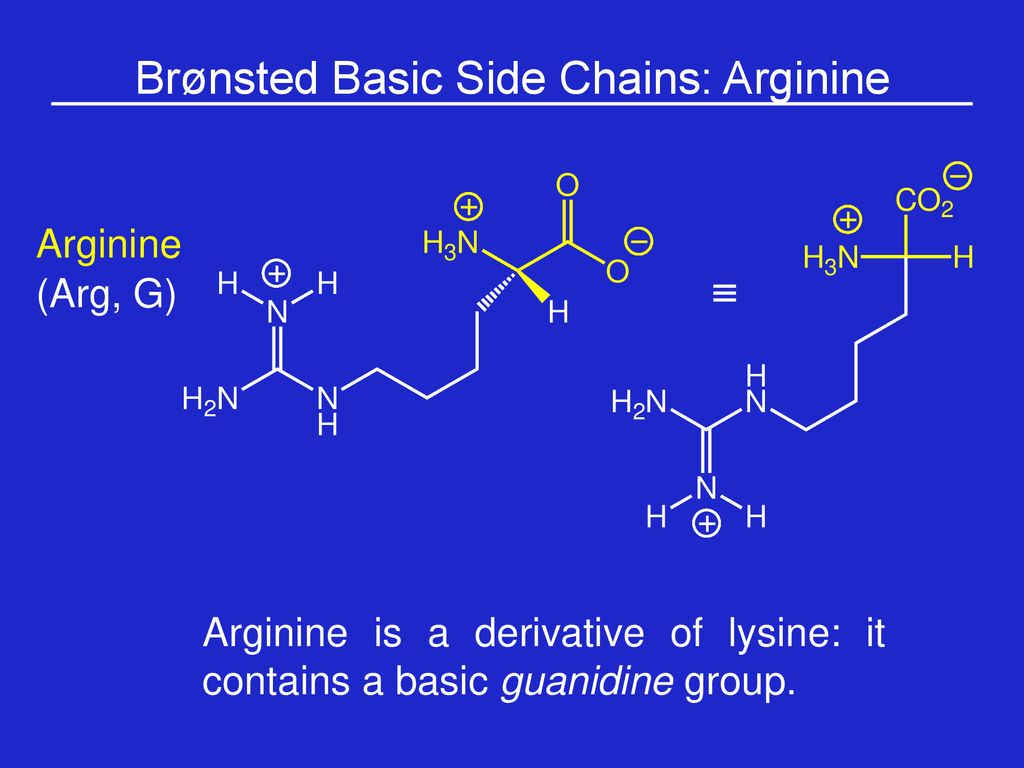



Week 1 Amino Acids Prof Sbw Ppt Download
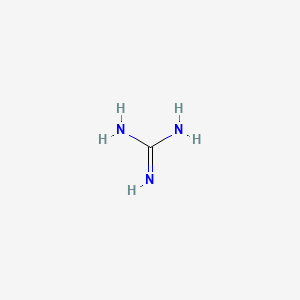



Guanidine Ch5n3 Pubchem




Recent Advances In Guanidine Based Organocatalysts In Stereoselective Organic Transformation Reactions Intechopen




The N Nitrosourea Of Szn Is Derived From An Intact Guanidine Group Of Download Scientific Diagram




Guanidine Synthesis Use Of Amidines As Guanylating Agents Baeten 16 Advanced Synthesis Amp Catalysis Wiley Online Library



1




Selective Functional Group Transformation Using Guanidine The Conversion Of An Ester Group Into An Amide In Vinylogous Ester Aldehydes Of Imidazole Sciencedirect
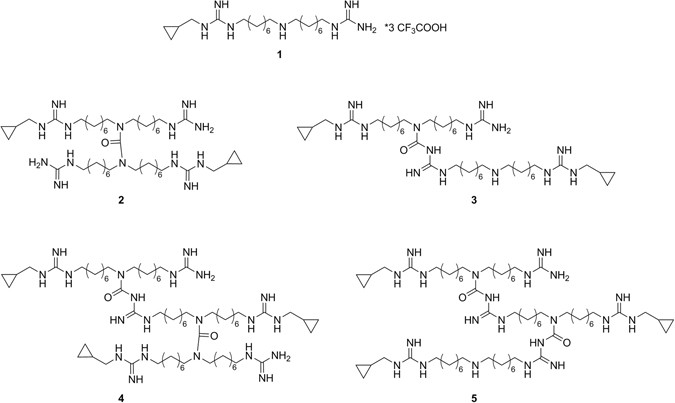



Identification Synthesis And Biological Activity Of Alkyl Guanidine Oligomers As Potent Antibacterial Agents Scientific Reports
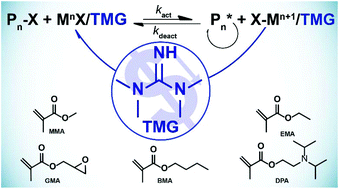



Guanidine As Inexpensive Dual Function Ligand And Reducing Agent For Atrp Of Methacrylates Polymer Chemistry Rsc Publishing




Aromatic Guanidines As Highly Active Binary Catalytic Systems For The Fixation Of Co2 Into Cyclic Carbonates Under Mild Conditions Catalysis Science Technology Rsc Publishing




Pdf Guanidine Motif In Biologically Active Peptides Semantic Scholar




Guanidine Wikipedia




Concise Synthesis Of Guanidine Containing Heterocycles Using The Biginelli Reaction Abstract Europe Pmc




Synthesis And Coordination Of A Neutral Phosphaguanidine And Comparison Of Its Basicity With A Guanidine
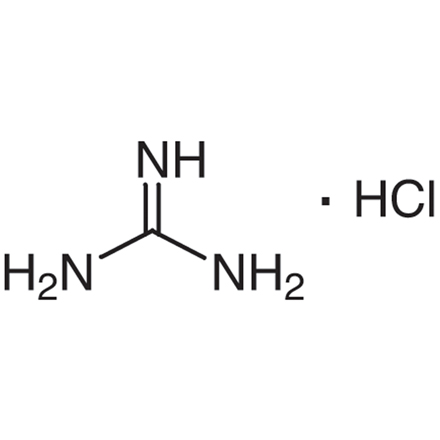



Guanidine Hydrochloride 50 01 1 Tci Europe N V
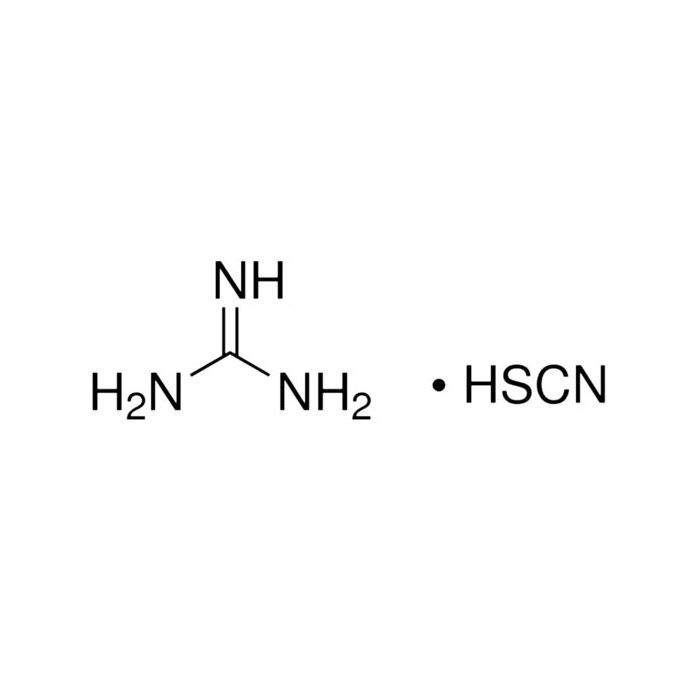



Guanidine Thiocyanate



1




Room Temperature Stable Multitalent Highly Reactive And Versatile Copper Guanidine Complexes In Oxygenation Reactions Springerlink




Pdf Amidines Isothioureas And Guanidines As Nucleophilic Catalysts Semantic Scholar



2
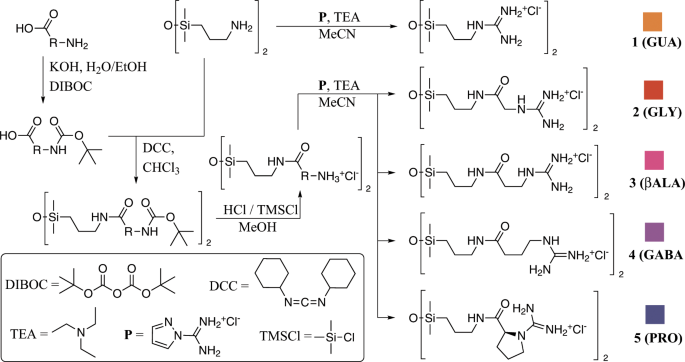



Exploring Ion Ion Preferences Through Structure Property Correlations Amino Acid Derived Bis Guanidinium Disiloxane Salts Scientific Reports




Guanidinium Salt An Overview Sciencedirect Topics




Thiocyanate Png Images Pngegg



Guanidine Wikipedia




Reaction Of A B Dicarbonyls With Guanidine Download Scientific Diagram




Derivatives Of The Triaminoguanidinium Ion 6 Aminal Forming Reactions With Aldehydes And Ketones
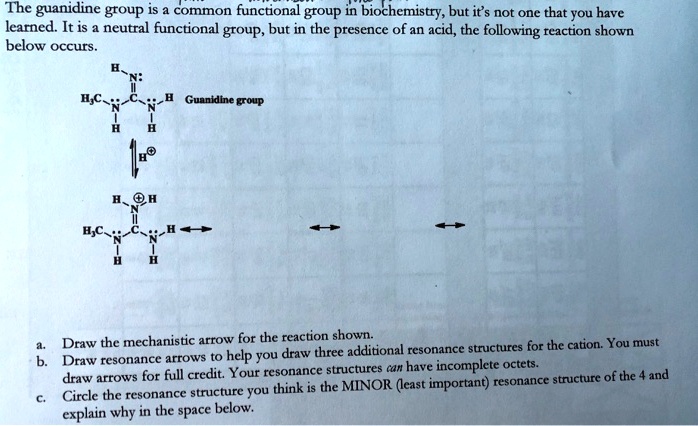



Z0eg3zaa3wsvem



Guanidinium Chloride Wikipedia



2



Illuminati References




Diaminosquarate Bioisostere For The Guanidine Moiety As A Novel Hiv 1 Download Scientific Diagram




Guanidinium Chloride Wikipedia
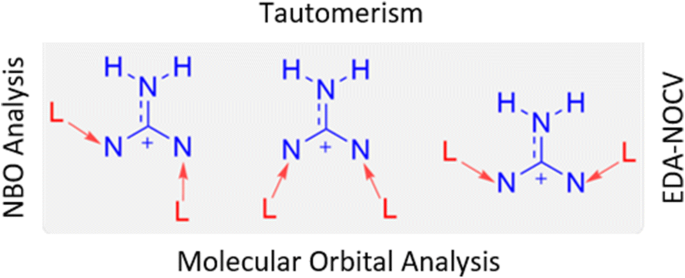



Quantum Chemical Study In Exploring The Role Of Donor Acceptor Interactions In 1 3 Bis Carbene Stabilized Guanidinium Cations Springerlink
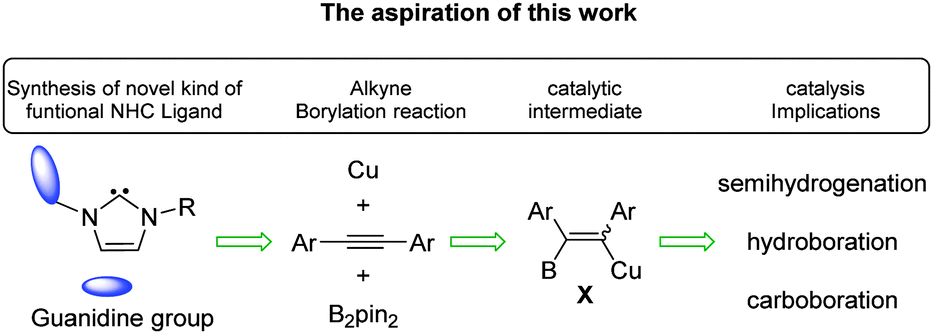



Synthesis Of A Guanidine Nhc Complex And Its Application In Borylation Reactions Chemical Communications Rsc Publishing Doi 10 1039 C4ccc




Guanidine And Guanidinium Cation In The Excited State Theoretical Investigation The Journal Of Chemical Physics Vol 141 No 7




Guanidine Youtube



1
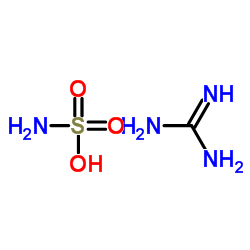



Guanidine Sulfamate Cas 2 Chemsrc
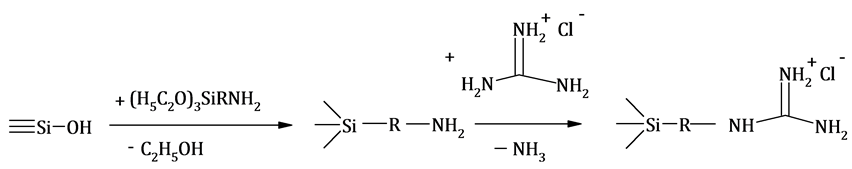



New Approach To Synthesis Of Silica With Chemically Bound Guanidine Hydrochloride For Preconcentration Of Metal Ions
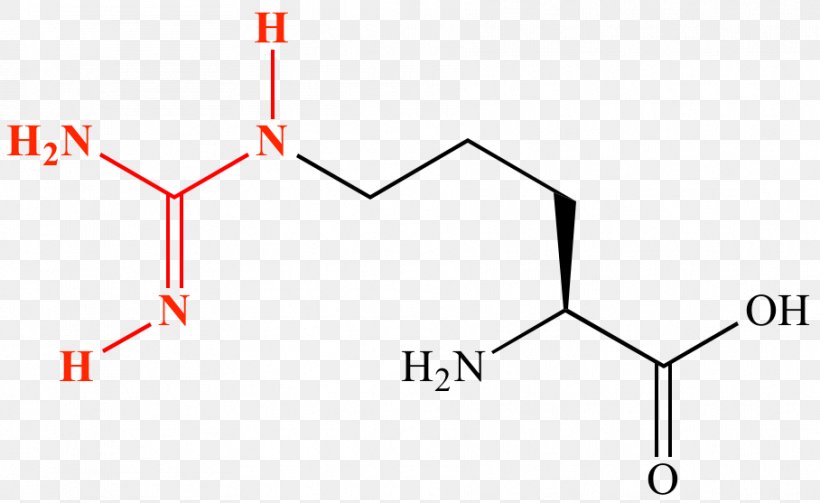



Amino Acid Amine Guanidine Protein Lewis Structure Png 908x557px Amino Acid Acid Alpha And Beta Carbon


